New test kits give answers within 45 minutes

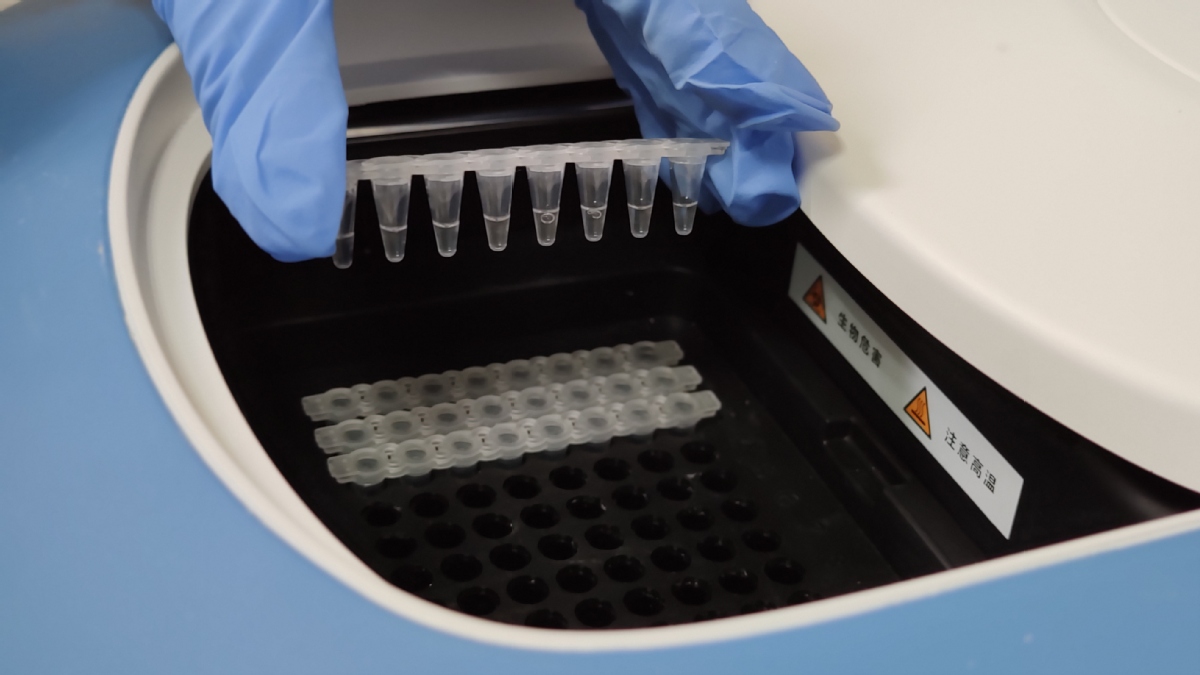
Zhang said, "From clinical practices to scientific research, we are finding new solutions to our diagnostic issues by optimizing our procedures and improving the test kits."
On March 31, China stepped up oversight of exports of diagnostic tools after several European countries raised concerns about their accuracy. The country now requires exporters to obtain a registration certificate from the NMPA and for approval to be granted by the importing nation.
About a week later, Chinese customs officers confiscated just over 1 million unregistered test kits after some exporters attempted to bypass the new regulation by categorizing their kits for the virus as "rapid test kits."
Jiang Fan, a senior inspector at the Ministry of Commerce's Department of Foreign Trade, said at a news briefing last week, "The ministry will partner with other related government bodies to enhance oversight of production, approval and customs clearance for medical products.
"We will severely punish anyone who tries to sell counterfeit goods or disrupt the market. There will be no exceptions."
Jiang also urged other countries to strengthen oversight of such imports, and strictly follow the instructions for use of medical products. Her comments followed recent reports that fake CE marking by third parties was becoming more widespread.