Phase II clinical trial for COVID-19 vaccine underway
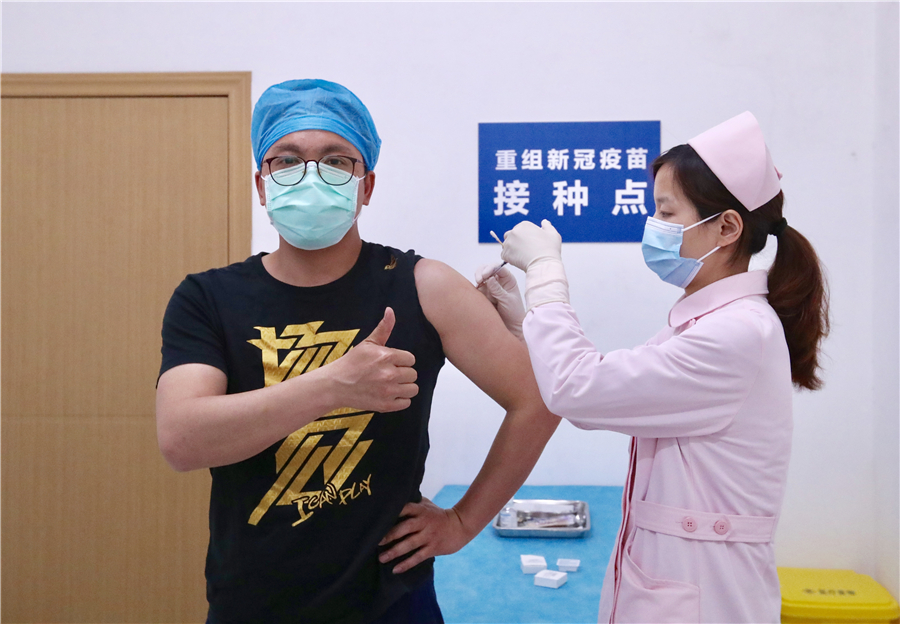
Chinese scientists have kicked off the second phase of a clinical trial of a COVID-19 vaccine in the hardest-hit city of Wuhan, in Central China's Hubei province, on Sunday.
It is China's first recombinant vaccine candidate for novel coronavirus entering Phase II of a human clinical trial, with 500 volunteer participants.
The eldest volunteer is 84-year-old Wuhan resident Xiong Zhengxing, who completed the vaccination on Monday morning, accompanied by his daughter.
The trial is led by the Jiangsu Provincial Center for Disease Control and Prevention and jointly conducted with the Hubei Provincial Center for Disease Control and Prevention and Zhongnan Hospital of Wuhan University.
The vaccine, developed by the Institute of Biotechnology, Academy of Military Medical Sciences, is constructed by genetic engineering methods and is used to prevent diseases caused by novel coronavirus infections.
The first phase of the vaccine clinical trial focused on its safety, while the second phase weighs more on its efficacy. Unlike the first phase, the second phase recruited more participants and introduced a placebo control group, according to Zhongnan Hospital.
Volunteer recruitment for the vaccine began on Thursday. It is China's first candidate for the virus that entered clinical human testing. The Phase I trial was conducted in March.
Please feel free to contact us by sending your questions to question@chinadaily.com.cn or commenting on China Daily app. We will ask experts to answer them.














